Recalibration of inhibitory control systems during walking-related dual-task interference: A Mobile Brain-Body Imaging (MOBI) Study
Abstract
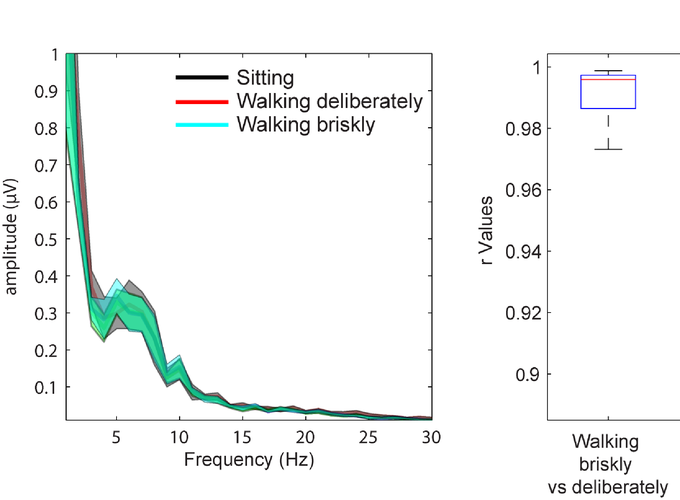
Walking while simultaneously performing cognitively demanding tasks such as talking or texting are typical complex behaviors in our daily routines. Little is known about neural mechanisms underlying cortical resource allocation during such mobile actions, largely due to portability limitations of conventional neuroimaging technologies. We applied an EEG-based Mobile Brain-Body Imaging (MOBI) system that integrates high-density event-related potential (ERP) recordings with simultaneously acquired foot-force sensor data to monitor gait patterns and brain activity. We compared behavioral and ERP measures associated with performing a Go/NoGo response-inhibition task under conditions where participants (N=18) sat stationary, walked deliberately or walked briskly. This allowed for assessment of effects of increasing dual-task load (i.e. walking speed) on neural indices of inhibitory control. Stride time and variability were also measured during inhibitory task performance and compared to stride parameters without task performance, thereby assessing reciprocal dual-task effects on gait parameters. There were no task performance differences between sitting and either walking condition, indicating that participants could perform both tasks simultaneously without suffering dual-task costs. However, participants took longer strides under dual-task load, likely indicating an adaptive mechanism to reduce inter-task competition for cortical resources. We found robust differences in amplitude, latency and topography of ERP components (N2 and P3) associated with inhibitory control between the sitting and walking conditions. Considering that participants showed no dual-task performance costs, we suggest that observed neural alterations under increasing task-load represent adaptive recalibration of the inhibitory network towards a more controlled and effortful processing mode, thereby optimizing performance under dual-task situations